The Mystery of Kuru
In the 1950s, a district medical officer working in the highlands of New Guinea observed a fatal disease among the people of the Fore (FOR-ay) tribe. The Fore people called this sickness kuru, which means "trembling in fear." After intially becoming unable to walk, victims of kuru lost the ability to swallow or chew. Drastic weight loss would inevitably lead to death. Today we know that kuru is one of several diseases in humans and animals caused by prion (PREE-on) proteins.
In 1957, a virologist who had studied several infectious diseases among remote peoples, came to New Guinea to study kuru. Carleton Gajdusek wanted to uncover the cause of this unique and always fatal disease. He searched for sources of toxins in the Fore's diet and environment.
He conducted epidemiological studies and sent samples of brain tissue to the United States to be studied by a neuropathologist. Because there was no sign of inflammation in the bodies or brains of the kuru victims, and because kuru tended to appear within certain families, Gajdusek at first believed kuru was an inherited genetic disorder.
In 1976 Carleton Gajdusek became co-recipient of the Nobel Prize in Medicine for his "discoveries concerning new mechanisms for the origin and dissemination of infectious diseases."

A doctor examines a boy with kuru in New Guinea.
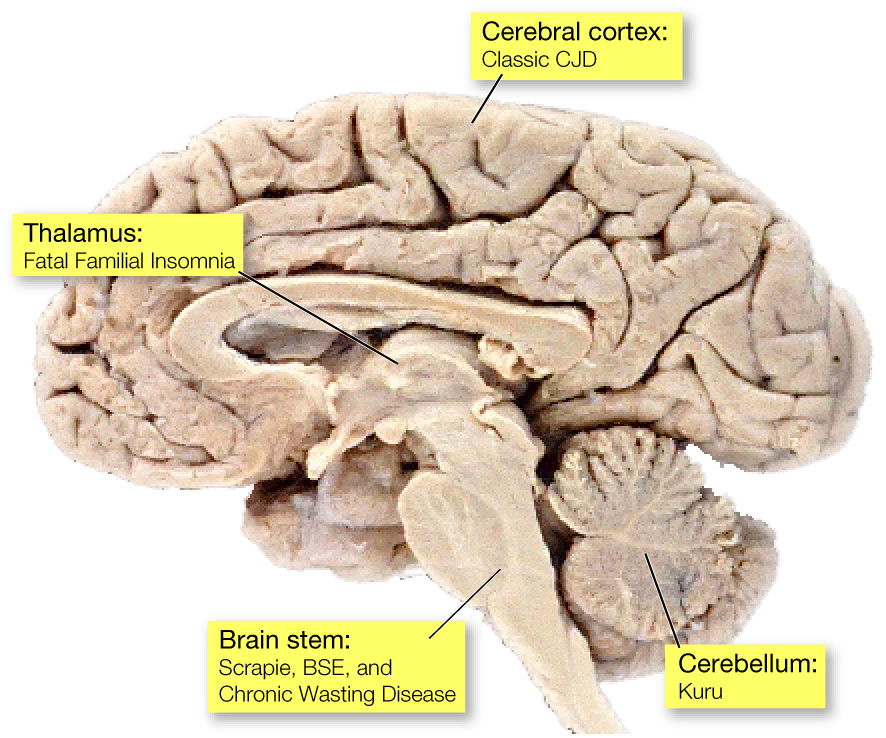
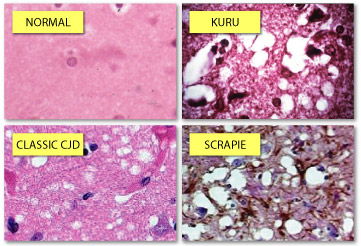
When you look at thin slices of kuru, classic CJD and scrapie brain tissue under the microscope, it is easy to see that they are full of holes. The holes form after misfolded prion proteins kill neurons in the brain. (Photo Credits: clockwise: Kunihiko Kobayashi; University of Iowa; Duke Medical School; University of California, Davis)
In 1959 Gajdusek's work came to the attention of William Hadlow, a research veterinarian who was studying a remarkably similar disease, called scrapie, in sheep. Like kuru, scrapie was a fatal disease that gradually destroyed the brains of sheep, leaving the brain full of holes and producing no immune response. And very importantly, scientists knew that scrapie was infectious.
The similarities between kuru and scrapie led Gajdusek to begin experiments to show that kuru could be transmitted to chimpanzees. He then went on to show that classic Creutzfeldt-Jakob disease (CJD), another spongiform disease in people, was also transmissible.
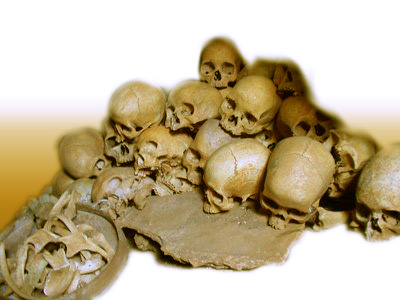
Ultimately, the rapid spread of kuru was linked to the Fore's funeral rituals: the Fore cooked and ate their dead relatives. This practice was only carried out by the Fore women and children, who lived apart from the men. This explains why men were rarely infected, and why cases appeared within families. The Fore quickly stopped eating their dead, and the spread of the disease stopped. Unfortunately, because of kuru's long incubation time, there are still a few kuru cases among the Fore each year. The people who come down with kuru today are in their 50s and 60s, which means that they have been harboring the disease ever since they ate infected tissue as young children.